
Insights+: EMA Marketing Authorization of New Drugs in December 2023
Shots:
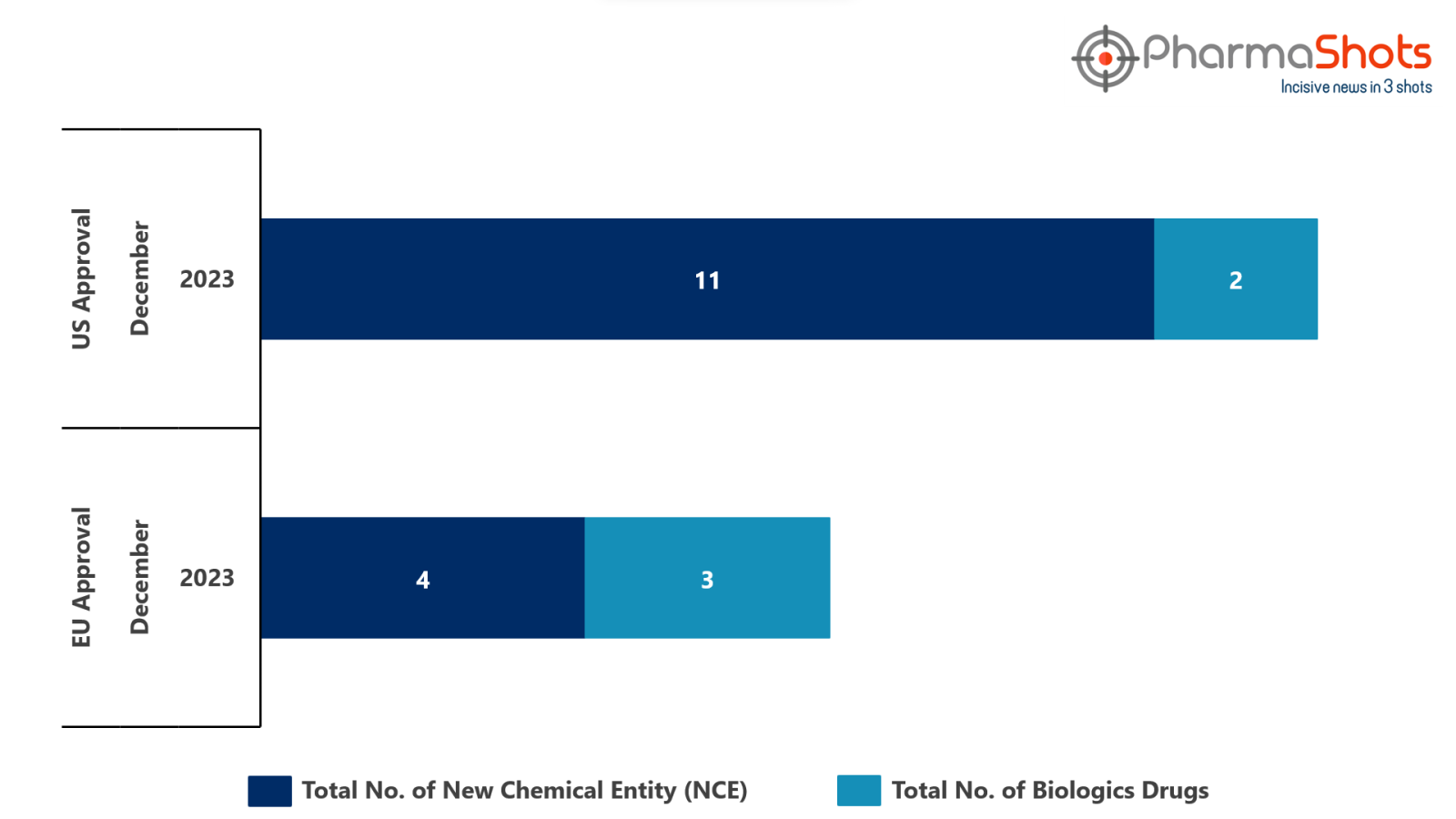
- The EMA approved 3 BLA while 4 New Chemical Entities in December 2023, leading to treatments for patients and advances in the healthcare industry
- In December 2023, the major highlighted drugs were Jempreli to treat dMMR/MSI-H Primary Advanced or Recurrent Endometrial Cancer and Zilbrysq for the treatment of Generalized Myasthenia Gravis
- PharmaShots has compiled a list of a total of 7 new drugs approved by the EMA in December 2023
Product Name: Jempreli
Active ingredient: Dostarlimab
Company: GSK
Date: Dec 11, 2023
Disease: Endometrial Cancer
- The EC has approved Jemperli + carboplatin-paclitaxel to treat dMMR/MSI-H primary advanced/recurrent endometrial cancer. This also converts its previous conditional approval into full approval for the same post-progression on a Pt-containing regimen
- The approval was based on part 1 of the P-III (RUBY) study assessing dostarlimab + carboplatin-paclitaxel followed by dostarlimab vs PBO. Part 2 assesses dostarlimab + carboplatin-paclitaxel followed by dostarlimab + niraparib vs PBO for primary advanced/recurrent endometrial cancer
- Results with a median follow-up of ≥25mos. showed a PFS of 72% with Jemperli added to CT. In a prespecified exploratory analysis for OS in the dMMR/MSI-H population, Jemperli with CT resulted in a 70% reduction in the risk of death vs CT
Product Name: Zilbrysq
Active ingredient: Zilucoplan
Company: UCB
Date: Dec 04, 2023
Disease: Generalized Myasthenia Gravis
- The EC has granted approval to Zilbrysq as an add-on to standard therapy for treating anti-AChR antibody+ gMG, valid across all EU member states along with Iceland, Liechtenstein, and Norway. The drug availability will begin in Q1’24
- The approval was based results from the P-III (RAISE) trial evaluating the efficacy, safety and tolerability of Zilbrysq (0.3mg/kg, SC, 12wks.) vs PBO in adult patients, randomized 1:1, with anti-acetylcholine receptor (AChR) antibody-positive gMG
- The results revealed that at wk12, Zilbrysq showed rapid, consistent and significant improvements in various patient- and clinician-reported outcomes among mild-to-severe anti-AChR antibody+ gMG adult patients
3. Santhera’s Agamree (vamorolone) Receives EC’s Approval to Treat Duchenne Muscular Dystrophy
Product Name: Agamree
Active ingredient: Vamorolone
Company: Santhera
Date: Dec 18, 2023
Disease: Duchenne Muscular Dystrophy
- Followed by the positive opinion from the CHMP, the EC has granted approval to the Santhera’s Agamree (dosing b/w 2 and 6mg/kg/day, for total of 30mos.) for the treatment of DMD patients (aged 4yrs. & +) based on results from the (VISION-DMD) and 3 other trials
- The results showed that Agamree neither reduced bone metabolism nor bone mineralization in the spine after 48wks. and recovered growth & bone health after switching from prednisone. Further data is being collected for its efficacy and safety
- Additionally, Catalyst Pharmaceuticals holds an exclusive license for Agarmee in North America & expects the US launch in Q1’24. Santhera anticipates the drug’s launch in Germany during Q1’24
Product Name: Ayvakyt
Active ingredient: Avapritinib
Company: Blueprint Medicines
Date: Dec 12, 2023
Disease: Indolent Systemic Mastocytosis
- Blueprint Medicines has received approval from the EC for Ayvakyt (avapritinib) to treat adult patients with indolent systemic mastocytosis (ISM)
- Following a positive opinion from the CHMP, the EC approved Ayvakyt based on data from the study (PIONEER) which demonstrated significant improvements compared to PBO in 1EP & 2EP, addressing overall symptoms & mast cell burden along with favorable safety profile
- Ayvakyt, a kinase inhibitor designed to potently and selectively target KIT D816V that is already approved by EC for ISM, adults with ASM, SM-AHN/MCL/GIST
Product Name: lazertinib + Rybrevant
Active ingredient: amivantamab-vmjw
Company: Janssen
Date: Dec 21, 2023
Disease: Lung Cancer
- The submission was based on the P-III (MARIPOSA) clinical trial evaluating lazertinib + Rybrevant vs osimertinib & vs lazertinib alone as a 1L treatment of patients (n=1,074) with locally advanced or metastatic NSCLC with EGFR ex19del or L858R substitution mutations. The 1EP of the study includes PFS & the 2EPs include OS, ORR, DoR, PFS2 & intracranial PFS
- As per the results of the trial, the study depicted a statistically significant & clinically meaningful improvement in PFS for lazertinib + Rybrevant vs osimertinib (23.7 vs 16.6mos.). Janssen presented these results at ESMO 2023
- Lazertinib is an oral brain-penetrant EGFR TKI that targets both the T790M mutation and activating EGFR mutations while sparing wild-type EGFR
6. Cidara Therapeutics Receives European Approval for Rezzayo to Treat Invasive Candidiasis
Product Name: Rezzayo
Active ingredient: Rezafungin
Company: Cidara Therapeutics
Date: Dec 22, 2023
Disease: Invasive Candidiasis
- The approval was led by the positive CHMP opinion & was based on the results from the P-III (ReSTORE) clinical trial evaluating the safety & efficacy of Rezzayo vs SoC (caspofungin) in patients with invasive candidiasis
- The results from the trial depicted a statistical non-inferiority for Rezzayo (QW) vs Soc (BID). These results were supported by the results from the P-II (STRIVE) clinical trial & an extensive nonclinical development program
- Rezzayo, an echinocandin, has received an ODD by the EMA & has been approved by the US FDA. Moreover, as part of its Sep 2019 collaboration with Mundipharma, Cidara will receive a milestone payment of ~$11.14M on Rezzayo’s EU approval
Product Name: Evkeeza
Active ingredient: Evinacumab
Company: Ultragenyx
Date: Dec 18, 2023
Disease: Homozygous Familial Hypercholesterolemia
- The extended approval came following the CHMP's positive recommendation for Evkeeza in Nov 2023 & was based on the P-III safety, tolerability, PK & efficacy evaluation of Evkeeza (15mg/kg, Q4W) in children aged 5-11yrs. with HoFH. The 1EP of the study includes the change in LDL-C at 24wks. & 2EPs include effect on other lipid parameters
- As per the results, children depicted a reduction in their LDL-C levels by 48% along with a significant reduction in other lipid parameters incl. levels of ApoB, non-HDL-C & total cholesterol
- Evkeeza, an ANGPTL3 inhibitor, received the initial approval by the EC as an adjunct to diet & other lipid-lowering therapies in adolescents & adults aged ≥12yrs. with HoFH in Jun 2021
*The report has been compiled as per available data
Related Post: Insights+: EMA Marketing Authorization of New Drugs in November 2023
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














